
Streamlining Pharma Trials and Expediting Time to Market
The pharmaceutical industry faces numerous challenges in bringing new drugs to market, including lengthy and costly clinical trials. While patient safety and efficacy remain top priorities, it is equally vital to expedite time to market in order to meet the growing demand for innovative therapies.
Fortunately, modern strategies and technologies have emerged that can help streamline pharma trials without compromising safety or efficacy. These include adaptive trial designs, real-world evidence, and digital technologies like electronic health records and mobile health apps.
In this article, I’ll investigate these modern strategies and technologies in-depth, providing insight into how they can be used to expedite pharma trials in the UK. I’ll aim to give an understanding of how your company can leverage these tools to accelerate time to market while ensuring the safety and efficacy of your products.
Adaptive Trial Designs
Adaptive trial designs are a modern approach to clinical trials that enable researchers to make changes in real time based on the data gathered during the trial. Unlike traditional clinical trials, which follow a predefined protocol, adaptive trials allow for modifications to be made to the study design, sample size, endpoints, and treatment regimens mid-study.
One of the main advantages of adaptive trial designs is that they allow for quicker decision-making. Researchers can analyse data as it becomes available and make adjustments to the study protocol accordingly. This makes them an excellent example of how pharma trials can be expedited in the UK, as well as reducing costs by minimising the need for additional studies.
Additionally, adaptive trial designs can help reduce the number of patients needed for the trial, making it more ethical and efficient.
Do Adaptive Trials Work?
Successful examples of adaptive trial designs in the pharmaceutical industry include the I-SPY 2 trial, which tested new therapies for breast cancer, and the REMAP-CAP trial, which investigated treatments for sepsis. In both cases, the adaptive design allowed researchers to identify promising therapies more quickly than would have been possible with a traditional trial design.
While adaptive trial designs offer numerous benefits, they do require careful planning and execution. The trial protocol must be developed in advance with input from all stakeholders, including regulators, to ensure that the study remains objective and scientifically valid. Additionally, the data collected during the trial must be carefully monitored and analysed to ensure that any modifications made to the study design are based on sound scientific evidence.

Real World Evidence (RWE)
Real-world evidence (RWE) refers to data collected from sources outside of traditional clinical trials, such as electronic health records, claims databases, and patient registries. RWE complements conventional clinical trial data by providing a more complete picture of the safety and efficacy of new therapies in real-world settings.
One of the main advantages of using RWE is increased generalizability. Clinical trials are often conducted in highly controlled environments that may not accurately reflect the broader patient population.
By supplementing traditional trial data with RWE, researchers can better understand how therapies perform in real-world settings and among diverse patient populations. Additionally, RWE studies can be conducted more quickly and at a lower cost than traditional clinical trials, allowing pharmaceutical companies to expedite time to market.
How does RWE Work in Practice?
RWE has been used in the pharmaceutical industry, including the use of claims data to demonstrate the effectiveness of new treatments for diabetes and the use of electronic health record data to assess the safety of new cancer therapies. In both cases, RWE provided valuable insights into the safety and efficacy of these therapies in real-world settings, allowing for faster regulatory approval and more efficient time to market.
While RWE can represent an excellent way for pharma trials to be expedited to market, it’s important to note that it’s not a substitute for traditional clinical trial data. Instead, RWE should be viewed as a complementary source of information that can help inform clinical trial design, support regulatory submissions, and provide post-market surveillance data.
Digital Technologies
Digital technologies, such as electronic health records (EHRs) and mobile health apps, offer numerous opportunities to streamline pharma trials and expedite time to market. EHRs allow researchers to access patient data in real time, improving the efficiency of data collection and analysis. Mobile health apps can be used to collect patient-reported outcomes, monitor compliance with treatment regimens, and provide real-time feedback to patients and researchers.
What Are the Benefits?
One of the main benefits of using digital technologies in pharma trials is improved patient recruitment and retention. By using mobile health apps and social media platforms, researchers can reach a broader patient population and attract patients who may not have participated in traditional clinical trials. Digital technologies can also help improve patient engagement and retention by providing patients with timely reminders and personalised feedback.
Advances in Oral Dose Drug Manufacturing
A key area of focus within this industry is the development and manufacturing of Oral Dose drugs. By embracing automation and innovation, this sector can explore new ways that pharma trials can be expedited in the UK.
A significant development in this space is the recent strategic collaboration between the Medicines Manufacturing Innovation Centre (MMIC) and CME Automation Systems, a leading provider of automation technology and a client I’m currently enough to be working with.
MMIC is a UK-based organisation specialising in the advancement of pharmaceutical manufacturing technologies. They are recognised for their innovative solutions that address the pressing challenges faced by the pharmaceutical industry.
What Does This Mean for Drug Manufacturing?
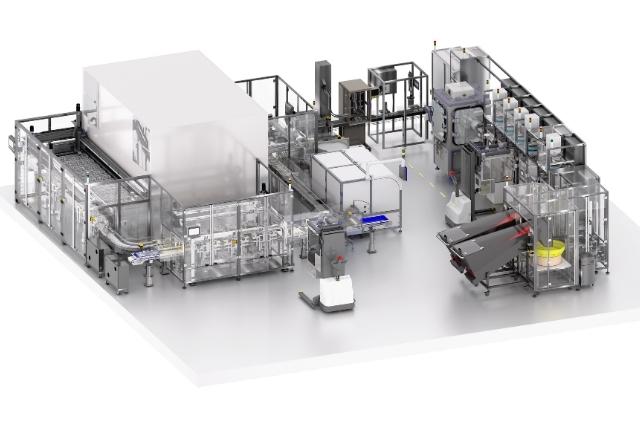
This partnership marks an important intersection of innovation in pharmaceuticals and automation technology. By leveraging CME’s state-of-the-art automation systems, MMIC aims to transform the production process of Oral Dose drugs. The objective is to devise an automated, efficient, and cost-effective method of production. Such a system could significantly reduce production time, minimise errors, and ensure product quality and consistency.
Innovation is at the heart of CME’s contribution to these developments. Their design solution for filling bottles with tablets or capsules vastly reduces the risk of cross contamination not only of the bottles themselves but also of the surrounding environment. By achieving this, their solution has decreased risk potential while improving output and efficiency significantly.
Find Out More About Speeding Up Clinical Trials of Oral Dose Drugs
If you’re eager to learn more about transforming the production processes for Oral Dose drugs, or if you want to stay up to date on the latest developments in clinical trials development then I can offer the support you need.
Contact me today for a detailed conversation, or view my news page for useful insights into a wide range of topics.

